4LIFE TRANSFER FACTOR ® TRI-FACTOR FORMULA®
4LIFE TRANSFER FACTOR® TRI-FACTOR® FORMULA PRODUCT DESCRIPTION
DIETARY SUPPLEMENT
———-
4Life Transfer Factor Tri-Factor Formula combines proprietary transfer factors and NanoFactor® molecules extracted from bovine colostrum and chicken egg yolk sources. These molecules contain antigen information which educates, enhances, and helps maintain immune system balance.
TECHNICAL DESCRIPTION
Transfer factors are molecules that communicate antigenic immunological information intercellularly and from a donor to a recipient. They support immune function through cell-mediated immunity (CMI). Transfer factors, which carry antigen-specific information to which all tested immune cells respond, are produced by mononuclear cells and serve to support and improve immune-mediated pathways. Mammalian transfer factors, including those of humans, are small molecules between 3,500 and 10,000 daltons. (1; 2) Transfer factors are polypeptides that consist of 40 to 44 amino acids (3) and have a conserved region and a variable region. From a molecular biological standpoint, these two properties are analogous to antibodies; however transfer factors’ functions of cell-mediated immunity (CMI) and nonspecific immunological activity differ almost completely from the functions of antibodies. The molecules that have a molecular weight of less than 3,500 daltons modulate immune response but they do not transfer delayed-type hypersensitivity (DTH). (1)
4Life’s transfer factors are sourced from the ultrafiltration of colostrum and from chicken egg yolks. (4; 5) The molecules obtained from the spray-dried ultra-filtrate of bovine colostrum are of two classes: the transfer factors present in the ultra-filtrate of ≤10,000 daltons and the nanofraction molecules that are present in the nanofiltrate of ≤3,500 daltons.
Transfer factors were first discovered in 1949 by Dr. H. Sherwood Lawrence when he demonstrated that CMI could be transferred from one individual to another by way of low molecular weight extracts of white blood cells. Transfer factors could transfer DTH of a specific form from a skin test positive individual to a skin test negative individual who subsequent to the transfer would skin test positive for that antigen. (6) In a subsequent study in 1955, Lawrence demonstrated that DTH could be passed serially, first from a skin test positive individual to a test negative individual, who became test positive, then six months later from the second individual to another test negative individual who became test positive. (7) At the time, antibodies were the focus of immune research and little was known of the importance of DTH and of the involvement of T-cells in immune response. Transfer factors promote wellness via cell-mediated immunity. These compounds are components of colostrum, an infant’s first meal. They bridge the generational gap by passing cell-mediated immunity from mother to infant.
BIOLOGICAL AND PHYSIOLOGICAL ACTION
Transfer factors’ preparations contain more than 200 different moieties of polypeptide molecules with a molecular weight of <10,000 daltons; each moiety potentially has a great number of epitope variations. These antigen-specific factors are synthesized in monocytes and stored in the cytoplasm or on the cell membrane. A significant body of evidence indicates that the primary biological function of transfer factors is to recruit and specifically sensitize previously uncommitted lymphocytes. These sensitized T-lymphocytes initiate the events of cell-mediated immunity, thereby promoting immunity not only at the site of antigen challenge but also throughout the body. (8) The effect of transfer factors on antigen-mediated immunity via B-cells is not completely understood; however, a clinical test has reported an increase in particular antibodies, such as IgA and IgG, during transfer factor administration.
Clinical studies have demonstrated that transfer factors’ unique ability to express DTH and promote cell-mediated immunity can be transferred from a sensitized donor to a non-immune recipient. (1; 9) This antigen-specific effect is well documented and is likely produced through activation of the CD3-antigen site of T-cells, increased macrophage activation, and interleukin production—which can also enhance natural killer cell function. (1; 10)
Although the exact mechanism of action is unknown, research has shown that transfer factors will bind to antigens. However, the antigen specificity that is «transferred» to recipients is mediated by T-lymphocytes. (3) Current structure function models propose that transfer factors have many, up to 818 possible, unique amino acids sequences, which allow transfer factors to be antigen specific (1). Transfer factors also have highly conserved regions that allow them to be administered across a species barrier without any loss of potency. In fact, research has demonstrated that bovine transfer factors are structurally analogous to human-derived transfer factors with equivalent physiological activity. (11) This is further supported by several studies, which used transfer factors extracted from bovine lymph nodes and colostrum to confer cell-mediated immunity to specific antigens in animals and human recipients. (12; 13)
Although most clinical trials with transfer factors have used parental administration, oral administration has also demonstrated successful transfer of DTH and cell-mediated immunity to recipients. (14) Dose response studies, which compare in various routes of administration, have been performed in both humans and animals. Results of these experiments refute any arguments that the acidic or enzymatic environment of gastrointestinal tract affects oral administration of transfer factors. (14)
CLINICAL AND EXPERIMENTAL STUDIES
Natural Killer (NK) Cell Activity
Peripheral blood mononuclear cells were isolated and pooled from several healthy donors. Sixty thousand cells were added to each well of 96-well microtiter plate. Various immune-modulating ingredients, including 4Life Transfer Factor® Tri-Factor® Formula, were added to select wells on the plate and the 48-hour incubation started. At end of the incubation period, 30,000 K562 cells were added to each well. MTT assay techniques were used to determine the cytotoxic index. The various 4Life Transfer Factor products resulted in cytotoxic indices of 80-98%. By comparison, mononuclear cells incubated with IL-2 for the same 48-hour period produced a cytotoxic index of 88%.
CD4 T-Helper Cell Research
Multiple studies were performed using the FDA-approved diagnostic CD4 T-Helper cell assay kit and/or a T-Cell Memory (CD8) assay kit under development by the same company. Similar to the NK cell research described above, these in vitro studies were performed on 96-well microtiter plates measuring ATP production via a luciferase-based luminescence reaction.
The CD4 assay utilized Phytohaemagglutinin (PHA)-stimulated cells isolated from whole blood via the use of Dynabeads™. An 18-hour incubation of these isolated, stimulated CD4 cells with 4Life Transfer Factor products resulted in a modulation of immune cell activity as exhibited by a decrease in Adenosine Triphosphate (ATP) production without a negative impact on cell viability. It is hypothesized that this reduction on ATP production is a result of a redirection in immune cell focus, essentially diminishing the distraction induced by the addition of PHA to the microtiter wells.
Salivary Secretory IgA (SIgA)-Preliminary Investigation
Twenty-four subjects naïve to transfer factor supplementation were enrolled in a small-scale, preliminary test. Twenty-one were included in the final analysis. Salivary samples were collected from each subject weekly at roughly the same time of day and day of the week. Saliva was collected over a 5-minute period via passive drool while subjects chewed on a piece of Parafilm™. The samples were put on ice and then frozen at -70°C until assay. The commercial Salimetrics™ salivary IgA assay kit was used for analysis. Subjects were given 4Life Transfer Factor® Tri-Factor® Formula at 2 capsules per day for two weeks and then transitioned to 4Life Transfer Factor® RioVida® Tri-Factor® Formula at 60 ml per day for an additional two weeks. At the end of the 4-week supplementation period, the group showed an average 73% increase in salivary secretory IgA (SIgA) production over their baseline value. Furthermore, none of the 21 subjects showed SIgA production rate less than their baseline value at the end of the test.
Wellness Research
A study conducted with 30 college students found that either l× 15 days or 2× 15 days (with a 2 weeks’ break in between) of transfer factor administered according to label dose helped them maintain their health. In both groups, transfer factor administration improved the number of CD8+ T-cells and NK cells to healthier levels. Particularly, those who took the product for 2× 15 days showed prolonged health maintenance and improvement of immune cell markers than those who took it for 15 days. Specifically, the maintenance of good health and improvement of immune cell markers remained for up to 3 months after stopping transfer factor administration in those who took the product for 2× 15 days, in comparison to 1 month in those who took the product for 1× 15 days. (15)
Safety
In a study of acute toxicity, rats were assessed for 14 days following a single gavage of 4Life Transfer Factor. Five female SD rats were each gavaged with a dose of 2,000 mg/kg. No treatment-related mortalities occurred and there were no clinical signs of toxicity. No significant difference in body weight occurred. No gross lesions were found at necropsy in any of the animals. Thus, acute toxicity is considered to be greater than 2,000mg/kg (human equivalent dose of approximately 320 mg/kg). (16)
Another similar single-dose oral toxicity study was conducted in mice. Six female Wistar mice each received 2,000 mg/kg via oral gavage and monitored for 14 days. No observable toxicity occurred as assessed by mortality, body weight gain, histopathology of brain, liver, kidneys and lungs, clinical signs of aggression, lethargy, breathing difficulties, diarrhea, mobility, and shivering. Thus, the no-observed adverse effect level was considered to be greater than 2,006 mg/kg in mice, which is equivalent to approximately 9.7 g/day in humans. (17)
Recent toxicity studies performed by an independent toxicology laboratory were conducted to evaluate the mutagenicity and genotoxicity potential of 4Life Transfer Factor. Mutagenicity was assessed by the Bacterial Reverse Mutation assay. Results revealed that 4Life Transfer Factor has no mutagenic activity at any of the concentrations tested. Genotoxicity was assessed by the Mammalian Chromosome Aberration test. Results demonstrated that 4Life Transfer Factor, tested up to the maximum recommended concentration, did not induce structural chromosome aberrations in this mammalian system. The laboratory concluded that 4Life Transfer Factor is considered not clastogenic in this system.
Another recent study performed by the same toxicology laboratory assessed the oral toxicity of 4Life Transfer Factor in rats. In this 14-day repeated dose study, male and female Wistar rats received by oral gavage 1050, 2100, or 4200 mg/kg body weight/day of 4Life Transfer Factor or placebo. Results revealed that no mortality occurred at any given dose. Clinical observations showed no adverse effect of 4Life Transfer Factor on behavior and physical condition of the animals. No abnormal body weight gain of food consumption were observed. Ophthalmological and hematological evaluations demonstrated no adverse effect by 4Life Transfer Factor. Similarly, no changes were observed in clinical chemistry, gross pathology, organ weight, and histopathology at any given dose. It was concluded that the no-observed adverse effect level was greater than 4200mg/kg in rats. This dose is equivalent to 40g/day in humans (18).
The use of transfer factors is contraindicated in people receiving immunosuppressive therapy, though actual interactions have not been documented. The use of transfer factors during pregnancy and nursing has not been evaluated.
HOW SUPPLIED
4Life Transfer Factor® can be found in the following products:
4Life Transfer Factor® Tri-Factor® Formula
4Life® Transfer Factor Plus® Tri-Factor® Formula
4Life Transfer Factor® RioVida® Tri-Factor® Formula
4Life Transfer Factor® Chewable Tri-Factor® Formula
4Life Transfer Factor® Classic
4Life Transfer Factor® Immune Spray
4Life Transfer Factor® KBU®
4Life Transfer Factor® Belle Vie®
4Life Transfer Factor® Cardio
4Life Transfer Factor® GluCoach®
4Life Transfer Factor® MalePro®
4Life Transfer Factor® ReCall®
4Life Transfer Factor Reflexion®
4Life Transfer Factor Vista®
Renuvo®
RiteStart® Men
RiteStart® Women
RiteStart® Kids & Teens
Pre/O Biotics™
PRO-TF®
REFERENCES
1. Fundenberg, H. and G. Pizza. 1994, Progress in Drug Research, Vol. 42, pp. 309–400.
2. Lawrence, H.S. and W. Borkowsjy. (1–3), 1996, Biotherapy, Vol. 9, pp. 1–5.
3. Kirkpatrick, C.H. 4, 2000, Mel Med, Vol. 6, pp. 332–41.
4. Hennon, W. and D. Lisonbee. s.l. U.P. Office, Editor., 2002, 4Life Research, LC: USA.
5. Wilson, G. and G. Paddock. S.l.: U.P. Office, Editor., 1989, Amtron, Inc. USA.
6. Lawrence, H.S, 4, 1949, Proc Soc Exp Biol Med, Vol. 71, pp. 516–22.
7. Lawrence, H.S. 2, 1955, J Clin Invest, Vol, 34, pp, 219–30.
8. Levin, A.S., L.E. Spitler, and H.H. Fundenberg. 1973, Annu Rev Med, Vol. 24, pp. 175–208,
9. Fudenberg, H. and H. Fudenberg. 1989, Ann Rev Plharmacol Toxicol, Vol. 29, pp. 475-516.
10. See, D, S. Mason, and R. Roshan. 2, 2002, Immunol Invest, Vol. 31, pp. 137–53.
11. Dwyer, John M. 1–3, 1996, Biotherapy, Vol. 9, pp. 7-11.
12. Wilson, G.B., R.T. Newell, and N.M. Burdash. 1, 1979, Cell Immunol, Vol. 47, pp. 1–18.
13. Radosevich, J.K., G.H. Scott, and G.D. Olson. 4, 1985, Am J Vet Res, Vol. 46, pp. 875-8.
14. Kirkpatrick, C.H. 1–3, 1996, Biotherapy, Vol. 9, pp. 13-6.
15. Klimov, V. and E. Oganova. in Euromedica Hannover 2004. Hannover, Germany : s.n., 2004. pp. 1.5–16.
16. Kabirov, K.K. 2009, unpublished: University of Illinois at Chicago.
17. Burbano, Z. and G. Sarmiento. 2013. Facultad de Ciencies Quirnicas, Universidad de Guayaquil, Ecuador.
18. Hirka, G et al. Toxi-Coop Zrt. Budapest, Hungary.
PRODUCT PHOTO
NOTE: These photos can be used only for identification by shape, color, and imprint. They do not depict actual or relative size.

The product samples shown here have been supplied by the manufacturer and reproduced in full color by PDR as a quick-reference identification aid. While every effort has been made to assure accurate reproduction, please remember that any visual identification should be considered preliminary. In cases of poisoning or suspected over dosage, the drug’s identity should be verified by chemical analysis.
Physicians’ Desk Reference
The Physicians’ Desk Reference (PDR) is a commercially published compilation of manufacturers’ prescribing information (package insert) on prescription drugs, updated annually. While designed to provide physicians with the full legally mandated information relevant to writing prescriptions (just as its name suggests), it is widely available in libraries and bookstores, widely used by other medical specialists, and sometimes valuable to the layman. The compilation is financially supported in part by pharmaceutical manufacturing corporations which create drugs listed within its pages. The 71st Edition, published in 2017, was the final hardcover edition. It weighed in at 4.6 pounds and contained information on over 1,000 drugs.[1] Since then, the PDR is available online (PDR.net), and has been integrated into some electronic health record (EHR) systems.
Since the late 20th century, a consumer edition has been offered at a much reduced price. Electronic editions are available on CD-ROM and the World Wide Web to subscribers. In 1984, Paul C. Kranz and Michael Grondin travelled to Oradell, New Jersey, and presented to Medical Economics (then-publisher of the PDR) a prototype developed by Grondin on a TI 99/4A computer of how a digital copy of the PDR would work and benefit clinicians. The idea originally conceived by Kranz was well received by the president and vice-president of IT and an agreement was struck to investigate. The result was the PDR on CD-ROM. The main edition is usable by determined laypeople in conjunction with a medical dictionary.
Por el 13er año, los productos 4Life Transfer Factor aparecen en el Physicians’ Desk Reference (PDR). La edición del 2017 es una herramienta de referencia para los profesionales de la salud e incluye información detallada de la línea de productos 4Life Transfer Factor® Tri-Factor® Formula.
Qué es el PDR? (Physicians’Desk Reference)
En muchas de mis presentaciones, sobretodo a nivel de profesionales de la salud, he mencionado una de las informaciones que me resultan más significativas acerca del valor y la validez terapéutica de los Factores de Transferencia elaborados por 4LifeResearch:
Su presencia y recomendación profesional desde el PDR.
Cuando esta información la ofrecemos en países de América tiene un valor evidente pero cuando hablo del PDR en Europa suele no entenderse la importancia de lo que estoy apuntando; así que que la mayor parte de las veces suele requerirse una explicación añadida sobre esta guía de referencia.
Por esa razón voy a dejar en este post la información completa sobre el PDR y así todo el mundo conocerá la importancia de que 4Life Transfer Factor® esté incluido en sus páginas de consulta para los profesionales de la salud.
PDR (Physicians’ Desk Reference) es la guía de productos de referencia donde cientos de miles de profesionales de la salud en Estados Unidos consultan con seguridad los complementos terapéuticos que recomiendan a sus pacientes. En este libro está toda la información de los medicamentos, suplementos alimenticios y hierbas que están actualmente en el mercado y que pueden recomendar a sus pacientes. En éste se encuentran importantes informaciones técnicas de las innovaciones en los tratamientos, la ciencia del antienvejecimiento, estudios científicos y nutrición, entre otras cosas informaciones de valor que aportan seguridad al profesional que las recomienda.
Este libro, esta guía de referencia terapéutica, se distribuye cada año desde hace más de 60 años y alcanza más de 475,000 consultorios de terapia. Según las estadísticas, nueve de cada diez médicos consideran el PDR como su fuente de consulta más segura para tener toda la información actualizada anualmente sobre medicamentos, complementos nutricionales y tratamientos.
Los productos de 4LifeResearch, el 4Life Transfer Factor®, han sido incluidos en el PDR, Physicians’Desk Reference for Nonprescription Drugs, Dietary Supplements, and Herbs, desde el año 2003.
Estamos en este momento ante la edición del PDR del año 2015, con la gama de 4Life Transfer Factor® incluida en sus recomendaciones para profesionales.
Esta es una de las garantías que ofrecen los productos del laboratorio de 4LifeResearch a través de su patente sobre el método de extracción de los factores de transferencia asimilables por vía oral y su efectividad comprobada en mantener el equilibrio del bienestar del sistema inmunitario.
Recomendamos entrar al link de abajo para inscribirse sacándose un código ya que podrán hacer con eso su compra directa desde la compañía.
La edición del 2015 provee información detallada sobre 4Life Transfer Factor® Tri-Factor® Formula y una descripción técnica de la línea de productos Tri-Factor® Formula. Adicionalmente, la publicación provee una sinopsis de varios estudios científicos en referencia a los productos 4Life. Este año también incluye la fotografía de 4Life Trasnfer Factor Tri-Factor Formula.
“Estamos encantados porque de nuevo han sido incluidos en esta prestigiada publicación”, comentó el Vicepresidente de Mercadotecnia y Desarrollo de Productos Chad Renshaw. “El PDR es uno de los medios de consulta mejor corroborados entre la comunidad médica. Se distribuye a 500 mil profesionales de la salud en todo Estados Unidos. El que los productos 4Life se incluyan en el PDR provee insuperable credibilidad a nuestros distribuidores cuando comparten los productos con sus clientes”.
Con nueve patentes internacionales y docenas más en trámite, un equipo interno de Investigación y Desarrollo, y la experiencia científica del Consejo de Ciencias Médicas, el compromiso de 4Life con la ciencia de los factores de transferencia, no tiene paralelo.
“La lista del PDR refuerza nuestra credibilidad científica y empodera a los distribuidores con una valiosa herramienta para compartir los productos 4Life”, dijo el Director de Servicios de Información de la Salud Brent Vaughan.
4Life cuenta con oficinas en cinco continentes para dar servicio a una red mundial de distribuidores independientes a través de la ciencia, el éxito y el servicio.
4Life Transfer Factor® Aparece Nuevamente Publicado en una Importante Guía de Referencia Médica
Este es el duodécimo año, que los productos 4Life Transfer Factor fueron incluidos en el prestigiosa Physicians’ Desk Reference® (PDR). Esta herramienta de referencia incluye una serie de servicios que le ofrece acceso a los profesionales de la medicina a información importante relacionada a la salud incluyendo servicios digitales de comunicación, PDR.net®, y mobilePDR®.
“Es maravilloso que nos incluyan una vez más en esta guía de referencia, que ha sido una fuente confiable para los profesionales de la medicina por varias generaciones, dijo el director general científico de 4life, David Vollme. “El PDR siempre ha ofrecido información innovadora de la salud”.
La edición 2016 ofrece información detallada acerca de 4Life Transfer Factor® Tri-Factor® Formula y ofrece una descripción técnica de la línea de productos Tri-Factor Formula.
“El PDR continua siendo un recurso científico par aprender y entender los productos 4Life Transfer Factor. Estamos emocionados de ser incluidos una vez más en este prestigioso recurso”, dijo el vicepresidente de mercadeo de producto de 4Life, Chad Renshaw. “El PDR es una de las herramientas más reconocidas en la comunidad médica. La inclusión de los productos 4Life en el PDR le da a los distribuidores una credibilidad inquebrantable cuando presentan los productos a los clientes”.
Quizá también te interese: 4Life Participa en un Evento Educativo de Venta Directa Realizado en una Importante Universidad
4Life refuerza su compromiso a la ciencia de los factores de transferencia con cuatro patentes en los Estados Unidos y 32 patentes internacionales, un equipo interno de Investigación y Desarrollo, y la contribución científica del Consejo de Ciencias Médicas.
Productos de 4Life Ganan una Vez Más un Lugar en una Importante Guía de Referencia Médica
Por décimo tercer año, los productos 4Life Transfer Factor® aparecen en el Physicians’ Desk Reference (PDR).
El PDR es una herramienta de referencia para los profesionales de la salud y esta edición de 2017 incluye información detallada acerca de la línea de productos 4Life Transfer Factor® Tri-Factor® Formula.
“Es un honor ser incluido en esta guía de referencia una vez más”, dijo el Dr. David Vollmer, Director General Científico de 4Life. “El PDR ha sido un recurso de confianza para los proveedores de atención médica por décadas y siempre ofrece información innovadora acerca de la salud”.
4Life refuerza su compromiso con la ciencia de 4Life Transfer Factor con cuatro patentes en Estados Unidos y 32 patentes internacionales, un equipo interno de Investigación y Desarrollo, y las contribuciones científicas del Consejo de Ciencias Médicas.
“El PDR es uno de los recursos más corroborados en el cuidado de salud hoy en día”, dijo Chad Renshaw, Vicepresidente de Mercadeo y Desarrollo del Producto de 4Life. “Se distribuye a miles de profesionales de la salud en todo Estados Unidos. La inclusión de los productos 4Life provee a los distribuidores una credibilidad inigualable al presentar clientes potenciales a la compañía”.
Los Factores de Transferencia, así como los productos Transfer Factor de 4Life, están incluidos en la guía de referencia médica (Physicians Desk Reference (PDR)) para fármacos disponibles sin receta y complementos nutricionales, desde el año 2002 hasta la actualidad, con información actualizada e incorporación de los nuevos productos. Esta guía, ha sido y es, en la actualidad, utilizada por los médicos estadounidenses desde hace más de 60 años, como la guía estándar para encontrar información selectiva de fármacos disponibles sin receta y complementos nutricionales indicados con mayor frecuencia.
Cada producto listado incluye información relevante sobre las evoluciones y estudios clínicos realizados sobre el mismo.
La información acerca de 4Life Transfer Factor incluye un resumen sobre las investigaciones sobre varios de los productos, demostrando el respaldo que proporcionan a los sistemas, estructuras y funciones del cuerpo.
FACTORES DE TRANSFERENCIA EN EL PDR DESDE EL 2002.
Salt Lake City, Utah (4 de diciembre de 2018) Los productos 4Life Transfer Factor® aparecen en el Physicians’ Desk Reference (PDR) por décimo cuarto año consecutivo. El PDR es una herramienta de referencia para los profesionales de la salud que incluye información amplia acerca de la línea de productos 4Life Transfer Factor® Tri-Factor® Formula.
El Director General Científico, Dr. David Vollmer comentó: “Es fantástico que los productos aparezcan una vez más en el PDR. Este es un recurso confiable para los proveedores de asistencia médica, el cual ofrece información innovadora acerca de la salud” …
4Life trabaja diligentemente para distinguirse a través de productos nuevos e innovadores. Los productos 4Life Transfer Factor están patentados, son exclusivos y se someten a pruebas rigurosas de control de calidad.
El Vicepresidente de Mercadeo y Desarrollo de Productos, Chad Renshaw, compartió: “El PDR se distribuye a miles de profesionales de la salud en todos los Estados Unidos. La inclusión de los productos 4Life en el PDR les da credibilidad a los distribuidores al compartir 4Life con sus prospectos”.
4Life tiene oficinas en 24 mercados para servir a una red global de distribuidores independientes y sus consumidores.
Para obtener más información:
Calvin Jolley
Vicepresidente de Comunicaciones
4Life Research USA, LLC
CalvinJolley@4life.com
La lista PDR (Physicians’ Desk Reference) es una guía de referencia que contiene información sobre medicamentos y suplementos.
Es utilizada para ayudar a los doctores en las decisiones de prescripción que dan a sus pacientes.
Es la guía de referencia más reconocida en Estados Unidos en donde se reparte a más de 475,000 médicos.
La lista PDR incluye información importante sobre el producto 4Life Transfer Factor® Tri-Factor® Formula.
Aquí puede encontrarse una sinopsis de varios estudios clínicos.
La documentación tiene bastante información técnica, pero algunos puntos a resaltar son:
Estudio de Bienestar
Uno de los estudios se hizo entre estudiantes de universidad a quienes se les suministró factores de transferencia por 15 y 30 días.
Las dosis se suministraron de acuerdo a las indicaciones en la etiqueta.
Se observó que el producto los ayudó a mantener su salud.
Los que lo consumieron por 30 días mostraron un beneficio prolongado.
Estudio de Longevidad
En un estudio realizado en hombres entre 55 y 73 años, se les suministró a los participantes 3 cápsulas al día.
Se les suministró 5 días a la semana por un período de 6 semanas.
A la sexta semana se les realizó una prueba conocida como el método de Kiev.
Esta prueba mide la edad biológica de las personas.
El resultado mostró que una reducción de aproximadamente 4 años.
Se observaron mejorías significantes tales como:
- Función cardiovascular
- Escucha
- Balance
- Capacidad pulmonar
- Habilidad para aguantar respiración
Seguridad de Consumirlo
Desde el descubrimiento de los factores de transferencia en 1949, no se han reportado reacciones alérgicas.
Tampoco se ha reportado ningún efecto secundario por el uso prolongado de 10 años o más.
Son contraindicados para las personas que reciben terapia inmunosupresora, aunque la interacción real no se ha documentado.
4Life Research LLC (USA) is the world leader in the development, research and production of Transfer Factors extracted from cow colostrum and chicken egg yolks.
The innovative technology, high product quality and proven performance and efficiency form the basis of the growing demand for the unique immune system correctors – Transfer Factors and Tri-Factors – the products of the new generation for powerful support and a significant improvement of an immune system functional condition.
All products have passed the appropriate registration and have the Guidelines of AMS and MHU.
4Life upholds not only the current GMP standards, but also standards of pharmacological industry outlined by FDA. 4Life Tri-Factors are included in the Guide for U.S. doctors (PDR) for over than 10 years.
4Life Transfer Factor Center in Ukraine is the official distributor, the organizer of the registration, researches and supplier of products in the domestic market since 2005. We are looking forward for cooperation!
We invite you to the stand of the company, where you can get exhaustive answers to the questions that interest you, consult, help to make the right choice.ç
Los productos Transfer Factor® de 4Life están incluidos en la Guía de referencia médica “Physicians’ Desk Reference” (PDR) del año 2005 para fármacos disponibles sin receta y complementos nutritivos
Sandy, Utah (14 de junio de 2005) – Por tercer año consecutivo, los productos Transfer Factor de 4Life fueron incluidos en la Guía de referencia médica Physicians’ Desk Reference del año 2005 para fármacos disponibles sin receta y complementos nutritivos. Los médicos se han basado en la Guía PDR durante 58 años para brindar la información más reciente sobre fármacos disponibles sin receta y complementos nutritivos. Actualmente la Guía PDR aún es considerada la guía estándar de complementos para los médicos y puede encontrarse en prácticamente todo consultorio médico, hospital y farmacia de los Estados Unidos.
La Guía PDR también se encuentra disponible para cualquier persona, brindándole acceso a un listado selectivo de fármacos disponibles sin receta y complementos nutritivos indicados con más frecuencia, lo que le garantiza una elección segura y adecuada. A pesar de que la Guía PDR no publica resultados de investigaciones exhaustivas, cada producto listado contiene información sobre las evaluaciones relevantes y estudios clínicos realizados para dicho producto. La información acerca de 4Life incluye un resumen de las investigaciones sobre varios productos 4Life Transfer Factor , demostrando el respaldo que 4Life Transfer Factor brinda a los sistemas, estructuras y funciones saludables del cuerpo.
El listado de la Guía PDR del año 2005 incluye el producto más reciente de 4Life, 4Life Transfer Factor RioVida(TM) así como también la familia completa de 13 productos diferentes de 4Life Transfer Factor . Estos productos están circundados por un amplio espectro de tecnología cada vez más sofisticada y ciencia avanzada, incluido el descubrimiento de las yemas de huevo como una fuente nueva de factores de transferencia y la obtención de una patente para las mismas, el desarrollo de la tecnología de Targeted Transfer Factor®, el incremento de la función de los linfocitos citolíticos naturales (NK), y la estabilización de estas moléculas mensajeras en un preparado bebible.
La inclusión de 4Life en la Guía PDR ayuda a establecer su posición de liderazgo en el desarrollo, producción y distribución de productos de respaldo inmunitario a base de factores de transferencia. Dado que cada vez más médicos y clientes reconocen la efectividad y respaldo que los productos Transfer Factor de 4Life ofrecen, la ampliación nacional e internacional se está convirtiendo rápidamente en un hecho frecuente.
PDR.net
Transfer factor is currently being listed in the Physicians’ Desk Reference. http://www.pdr.net/drugpages/productlabeling.aspx?mpcode=30651060 — Preceding unsigned comment added by 202.156.10.11 (talk) 02:39, 9 December 2011 (UTC)
- Which itself adds nothing and at the bottom it states the claims were not FDA-evaluated. Removed. WLU (t) (c) Wikipedia’s rules:simple/complex 02:23, 12 December 2011 (UTC)
- Physicians’ Desk Reference® (PDR®)is the most trusted and commonly used drug information reference. This source is important as a reference to transfer factor information for the public to be aware of. Please refer to http://www.pdr.net/webpages/aboutus.aspx for more information on PDR. — Preceding unsigned comment added by 202.156.10.11 (talk) 02:40, 23 December 2011 (UTC)
- Yeah, still no. The PDR still contains little but summaries of animal research and a tiny study of 13 people. We don’t include primary studies, even if listed in a secondary study – particularly when we have to add the words «in rats» to the end. Just noting that the PDR mentions transfer factors adds nothing to the page. Removing the observation that TF are unproven in the treatment of any condition is downright inappropriate. WLU (t) (c) Wikipedia’s rules:simple/complex 03:13, 12 January 2012 (UTC)
- Physicians’ Desk Reference® (PDR®)is the most trusted and commonly used drug information reference. This source is important as a reference to transfer factor information for the public to be aware of. Please refer to http://www.pdr.net/webpages/aboutus.aspx for more information on PDR. — Preceding unsigned comment added by 202.156.10.11 (talk) 02:40, 23 December 2011 (UTC)
The most recent paper of any quality is http://www.ncbi.nlm.nih.gov/pubmed/10949913 from 2001.
The PDR is not the end all be all of medical information for a research molecule. You are conflating the vernacular «transfer factor» with the research term. Read the very first citation provided and look at the picture imaged – they reference a form a transfer factor coming from lymphocytes. However, when you go to the PDR reference you link to it clearly states «transfer factors are sourced from the ultra-filtration of colostrum and from egg yolks.». However, there is little to no evidence that colostrum or egg yolks have high concentrations of lymphocytes. It is not impossible that colostrum of egg whites could contain products derived from cellular lysates, but it is unproven. Even the drawing is not full accurate since cells do not secrete TF – if they did you could pick TF up in serum and not even need the cells to «transfer» the effects. There is a line of research involving cellular lysates and a parallel, but different, line researching the «commercial product known as transfer factor». So the PDR is going to refer solely to the commercially sold product, that has no evidence supporting it, but the cellular lysate research of the past is a distinctly different subject – and deserves to be treated as such.
New sources?
The most recent paper of any quality is http://www.ncbi.nlm.nih.gov/pubmed/10949913 from 2001.
There don’t seem to be many new sources that really address transfer factors. The newest review article seems to be from 1993. It’s like the interest mostly died. WLU (t) (c) Wikipedia’s rules:simple/complex 18:15, 16 February 2010 (UTC)
- The above summary is entirely incorrect, as a quick search of PubMed.gov for «transfer factor», «colostrinin», «proline rich polypeptides», or «leukocyte extract» will reveal. Indeed, the newest review paper is a 2009 article by Aaron White from Duke University. He also published a book on transfer factors that year. Four papers on the use of colostrinin (transfer factors) for Alzheimer’s disease were published in 2009. Interest has not «died» but is growing. Interest among consumers for products containing transfer factors has also increased, though it is important to note that the sale of transfer factors through multilevel marketing companies and research on transfer factors are separate issues. —Preceding unsigned comment added by Aaronmwhite (talk • contribs)
-
- Are you that Aaron White? Citing your own work, particularly a self-published book [1], [2], is a conflict of interest. Is the «review paper» the one from Explore! magazine? I couldn’t find the paper on pubmed or google scholar. Please stop adding massive dumps of primary source material – the preference is for secondary, medically-reliable sources that summarize research in the form of review articles and meta-analyses. Even if it is probable that these materials could be useful, it is apparent that they are not used as a main form of treatment. Per our policy on undue weight we are supposed to represent the status of the field as seen by the relevant experts, and based on WebMD, Quackwatch and the Memorial Sloan–Kettering Cancer Center, transfer factors are not seen as primary treatment options. The Cochrane Collaboration doesn’t reference them as a form of treatment (or even as an investigation topic [3]).
-
- What’s really missing are randomized controlled trials indicating TF is effective in the treatment of any condition, and similar evidence demonstrating TF is a mainstream modality. If it’s not a mainstream modality, using wikipedia to promote it as such is inappropriate. Until mainstream practice adopts the use of transfer factors, it should be portrayed as a fringe theory with minimal mainstream support. WLU (t) (c) Wikipedia’s rules:simple/complex 21:01, 16 February 2010 (UTC)
-
-
- Here’s a recent review paper from 2007. Berrón-Pérez R et al. Indications, usage, and dosage of the transfer factor. Rev Alerg Mex. 2007 Jul-Aug;54(4):134-9. Here’s another from 2008. White A. Why vaccines are not the answer – the failure of V520 and the importance of cell-mediated immunity in the fight against HIV. Med Hypotheses. 2008 Dec;71(6):909-13. Superjack4life (talk) 06:16, 17 February 2010 (UTC)
- Medical Hypotheses is worthless as a source – it’s not a «proof», experiment or review article. The sole criteria for inclusion is a coherently expressed idea. That’s neither a review article, nor a proof, and shouldn’t be used. I’ll look at the review article.
- The MSKCC page doesn’t support TF being used for herpes or CFS – both are based on single studies. The herpes study showed changes in secondary blood indicators (and closes with the summary «However, CD4 counts in the transfer factor group still remained below the levels for healthy individuals.») and this isn’t an indication of even clinical efficacy. The CFS study examined similar indicators of improvement for possible, prospective, hypothetical causes of CFS (the actual cause of which is still very much in question). WLU (t) (c) Wikipedia’s rules:simple/complex 12:12, 17 February 2010 (UTC)
- Here’s a recent review paper from 2007. Berrón-Pérez R et al. Indications, usage, and dosage of the transfer factor. Rev Alerg Mex. 2007 Jul-Aug;54(4):134-9. Here’s another from 2008. White A. Why vaccines are not the answer – the failure of V520 and the importance of cell-mediated immunity in the fight against HIV. Med Hypotheses. 2008 Dec;71(6):909-13. Superjack4life (talk) 06:16, 17 February 2010 (UTC)
-
I’m adding this link to Sloan-Kettering’s website, as they address the efficacy of Transfer Factor and it is definitely not all bad. In fact they state that there is efficacy in specific clinical situations. Anyone who has read these discussions would probably have interest in reading this:
http://www.mskcc.org/mskcc/html/69399.cfm
Thanks. —Preceding unsigned comment added by 75.51.173.67 (talk) 16:50, 14 October 2010 (UTC)
Transfer factor research vs transfer factor marketing and sales
More research (1000+ papers) has been published on transfer factors in reputable scientific and medical journals than most other immunomodulators. They have powerful, demonstrable effects on immune system activity, cell counts, etc. At the same time, a few companies are aggressively marketing transfer factors as cure alls for diseases. There IS evidence that transfer factors affect immune system function. There IS NOT sufficient evidence to back up the widespread claims made by these companies and their sales force. Importantly, the activities of these companies should be viewed as separate from the science on transfer factors. —Preceding unsigned comment added by Aaronmwhite (talk • contribs) 20:52, 16 February 2010 (UTC)
- Until mainstream medicine adopts the use of transfer factors as a useful, proven treatment modality, the article should be short and based on secondary (i.e. review) sources rather than primary in vitro or single studies. WLU (t) (c) Wikipedia’s rules:simple/complex 21:01, 16 February 2010 (UTC)
Discussion of recent changes
WLU – may I ask what qualifies you to claim any level of expertise on cellular versus colostrum transfer factor? It is clear you have not even read the citations you seem to «tolerate» on the page LCDR IAM (talk) 02:54, 13 January 2013 (UTC). — Preceding unsigned comment added by LCDR IAM (talk • contribs) 02:50, 13 January 2013 (UTC)
- Please see WP:MEDRS for what qualifies to make a medical statement on wikipedia. Also note that qualifications are irrelevant for editing wikipedia, what matters is the ability to verify information using reliable sources. Please note that in particular, primary sources (i.e. the results of individual experiments) are not adequate to make medical or treatment claims on wikipedia; though I am not an expert, from what I have seen there is no real acceptance of transfer factors as a treatment for any condition.
- I have left a note at WT:MED to attract attention to this page. WLU (t) (c) Wikipedia’s rules:simple/complex 17:30, 13 January 2013 (UTC)
No one is making medical recommendations. I specifically outline that there is no connection between the cellular product listed in the first section, and the dietary supplement. The information as you have provided is flatly incorrect. THe companies sell their «product» based on false equivalence between the work from Dr Lawrence and their claims. By not separating the two you are playing right into that. There is no mention of medical treatment recommendations – this is a scientific comment. I could say Interleukin 1 improves response to viruses and it would not be a «recommendation» for someone to go get an injection – nor could they even do so outside of a research study. You wanted newer references and were provided them – and, as for all scientific information, multiple «individual» experiments are used to . I AM an expert on the science of Transfer Factor the cellular molecule working at the NIH and your page, as formulated, plays into the nonsense claims of companies by giving the impression they are the same. They are two distinctly different items and need to be treated as such. I hope and pray your «flagging» gets someone to look at this as I would welcome any and all opportunity to outline that either you need to make two separate pages (one for «researched lymphokine» and one for «dietary supplement»). — Preceding unsigned comment added by LCDR IAM (talk • contribs) 17:53, 13 January 2013 (UTC)
- We have to be careful in what sources we use, and how we state it. The use of individual primary studies goes against WP:PSTS, WP:WEIGHT and WP:MEDRS. I note that changes made to the side effects section were not supported to the cited reference as well. As an «expert on the science of Transfer Factor», you have no special rights or privileges on this page; Wikipedia is a collaborative project, and you have to work with other editors here. As an expert, you should be able to provide good high quality sources and discuss them here. I would suggest making suggestions here on the talk page for changes to be discussed, rather than making whole-sale changes that appear to be contentious. Yobol (talk) 19:46, 13 January 2013 (UTC)
I understand that. But I am frustrated by another user simply overriding all edits without any reasoning other than «no it shouldn’t». LCDR IAM These are peer reviewed journals on a scientific review. Please look at the article on IL-1B. The «secondary source» as you would call it it basically a blog with a list of published papers. There is no systematic review as is being suggested. The comments on human derived TF on WebMD has no source itself – but that being said, please read the WebMD page. You will not they clearly state TF is «taken from a human or animal that has already developed protection (immunity) against a certain disease». Contrast this with with PDR reference that states it comes from milk and eggs. These are clearly not the same thing and I am adjusting the article to reflect that. LCDR IAM (talk) 20:11, 13 January 2013 (UTC)
-
-
- If you are a genuine expert interested in improving the page through wikipedia’s processes, this is a good thing. However, you must be aware of the rules and constraints; wikipedia is not like a scholarly journal or personal webpage. For one thing, original research is not permitted. Nor is attempts to predict what will be successful (even on the basis of expert knowledge. This is not the place to promote an idea or product.
- If you are interested in improving the page within the scope of the policies and guidelines, I will enumerate my objections to the current page and ask, as a demonstration of good faith, to remove them.
- There are several primary sources. Wikipedia is based on secondary sources, particularly for medical pages; please review WP:MEDRS. Primary sources should only be used for very good reasons, and I do not see the reason for including sources from 1980, 1996 or even 2002. If this is an active area of research, it should be easy to find recent review articles or scholarly books discussing the topic. This means that references 5 and 9 at minimum are inappropriate and should be removed. Conference proceedings are also not MEDRS, so 11 should be removed as well.
- Please populate the footnotes that remain. The fastest and easiest way to do so is to find the relevant publication on pubmed and use the pubmed number in the {{cite pmid}} template. The model for this is, in the edit pane, place {{cite pmid |PMID}} between <ref></ref> tags, replacing PMID with the actual number as appropriate. This will result in an incomplete template that it says will be populated soon. It lies. Click on the «jump the queue» hyperlink and the ‘bot will do the rest for you.
- There is considerable unsourced text. Per WP:PROVEIT, all challenged material must be verified by reference to reliable sources. If you are an expert in the area, I would expect you to have both a personal library and knowledge of the subject matter such that this is a trivial task for you.
- Please incorporate wikilinks to concepts that are not readily understood by nonspecialists. This includes things like varicella (which is actually a redirect so please link to chickenpox instead) and candida.
- Please correct the capitalization of the words used; wikipedia uses sentence case, so only capitalizes the beginnings of sentences and proper nouns.
- Please build a proper body; currently most of your edits have been to the lead, which is meant to summarize the whole article.
- Please correct the text so that it is not misrepresenting the source. As an example your edit removed the side effects verified by the WebMD article (which might be a neutrality problem, I can see no reason to remove it), and makes it look like the WebMD article discusses the use of TF in and out of clinical trials, which it does not.
- If you are sincere about improving the page, these items need to be addressed. I may not be a researcher working on transfer factors, but I know wikipedia. Please address them. I am more than happy to provide advice or suggestions if you are unclear on how to meet any of our policies or other editing requirements. WLU (t) (c) Wikipedia’s rules:simple/complex 19:51, 13 January 2013 (UTC)
- No original research was presented. Only information from sources that are peer reviewed. I specifically state that people should be aware there is no evidence for the dietary supplement and state that the human derived should not be done outside of a research setting. Thus I would politely contend that allowing the page to stand is promoting a product by falsely conflating the science with the supplement.
- If allowed I will remove them. I did place studies from 2010-2012 before they were deleted. But keep in mind that many scientific ideas are not repeated yearly. I.E. IL-1B causes fever – but the papers stating that are from the 1950’s. But since that was decided back then, I doubt you would have a paper researching if IL-1B causes fever in 2012.
- I will gladly do so if allowed. All of the articles cited are from pubmed.
- Understood. I admit I am less familiar with inserting redirects and so forth. But simplifying the langue and putting in more citations would not be a problem if I am told that I will be allowed to do so.
- Understood. But I will need to introduce the idea in the leads that there is a research molecule under that name, and a dietary supplement – but then will discuss each separately in the body.
- Was not my intention to do so. I can correct this but can also make additional citation on possible side effects. Keep in mind that these will be old articles and case reports. I.E. they will involve a published incidence of adverse effects.
- If you provide me confirmation that you will work with me on fixing these issues so the page is as accurate as possible and does not fail any of Wikipedia’s policies, I will be grateful to assist in improving this page. LCDR IAM (talk) 20:11, 13 January 2013 (UTC)
-
┌─────────────────────────┘I will flag some of the areas I consider to be problematic according to WP:OR. It’s possible your use of sources is a little different than ours. I had never seen any discussion of the conflation between supplement and molecule, if you can verify this information with a specific source, that would be very helpful. Regarding old sources – we have no aversion to «first» sources, classic ones where a compound is identified or whatnot. But science generally marches on – if a substance was proven to do something 20 years ago, this should be discussed in review articles, novel experiments, textbooks and the like. While we might cite the source from 1992, most of the information on the page should come from much more recent developments.
Let’s start with the current version and move from there. I suggest LCDR IAM do the actual editing (I don’t need the practice) and we should be OK. The best thing to do is to start with the body, clarify and expand ideas there, then write the lead. It’s easier, and a better page. If the body reflects an idea, particularly an important one, then it should/will be reported in the lead. Let’s start a new section to discuss below. WLU (t) (c) Wikipedia’s rules:simple/complex 20:44, 13 January 2013 (UTC)
Sources
I think the first course of action is to find high quality secondary sources discussing this topic. Most of the articles discussing this date back decades, which gives me pause. A quick pubmed search did not find any recent reviews in the past 15 years. A few college level Immunology textbooks I leafed through also did not have this term. Are there any recent good sources for this? Yobol (talk) 20:25, 13 January 2013 (UTC)
- I’m hoping LCDR IAM can provide some; when I looked into it a bit a couple months (possibly a year ago) I noted there were very few sources and few MEDRS suitable for a wiki page; a more recent search turned up a couple. We’ve got an expert, let’s try to retain them. WLU (t) (c) Wikipedia’s rules:simple/complex 20:33, 13 January 2013 (UTC)
- Since TF is from blood, most clinical trials stopped the day after the discovery of HIV and HepC for obvious reasons. So almost all of the clinical studies will be old. I can make sure to highlight this better. There are a few newer ones, but the methods are less impressive. So, there are good sources, but I will admit most are going to be old when discussing clinical trials. So TF never made the textbooks because the human trial research spiked then faded quickly.
- Believe me, I do not hold TF in the same light as Aaron White. My goal is for people to know that the supplement can make no legit claims to even being TF, and that anyone that claims to have real TF got it from someone or some animal’s blood. If anything the page I am trying to construct will have people less likely to run out and buy it, but hopefully will leave anyone looking for the science more informed. LCDR IAM (talk) 20:42, 13 January 2013 (UTC)
- We probably should be avoiding clinical trials (i.e. primary studies) for now. We should be focusing on review studies, textbooks, and other secondary sources. I’m having trouble finding even one good place to start as an overview. Yobol (talk) 20:44, 13 January 2013 (UTC)
- It may be that we do not have adequate secondary sources. In situations like this in the past, instead what I have done is carefully and judiciously drawn from the literature review of primary sources. If there is nothing on TF in secondary sources, then that suggests the page will of necessity be quite short. LCDR IAM, please note that this is the reality of wikipedia – we are always behind the cutting edge; we do not identify promising areas of research or possible uses of compounds, we just note what is found in mainstream sources. Ideally the most current ones, but we are quite strongly limited in what we can neutrally express. If most people don’t think TF are of use, if TF aren’t used by mainstream hospitals, then the page should reflect TF as a research molecule, not a treatment. WLU (t) (c) Wikipedia’s rules:simple/complex 20:54, 13 January 2013 (UTC)
- We probably should be avoiding clinical trials (i.e. primary studies) for now. We should be focusing on review studies, textbooks, and other secondary sources. I’m having trouble finding even one good place to start as an overview. Yobol (talk) 20:44, 13 January 2013 (UTC)
-
-
-
-
- There are a few review articles, but honestly they are a list of the primary sources condensed. I guess I’d argue that multiple trials showing the same thing should be okay – however, I understand and respect your concern. If the issue is that you want no mention of treatment benefits that are not in a textbook, that might be hard and I am willing to leave all that out. Thus, I can remove all of that and just try to focus on clearly stating what is known about the lymphocyte product and that the supplement is not the same thing. I.E. mention that there was evidence of benefit, but none was more than a pre-clinical study and should not be looked upon and a secondary source (I would thus not list any disease specifically). Point out that the science of TF as a immune molecule is on going, but died out years ago. Lastly, point out that the dietary supplement is different and can make no viable claims to being the same thing (which is what I most want to highlight b/c I neither want the molecule to get a bad name from the supplement nor the supplement to ride the coattails of the molecule – even if said coat tails are decades old). This should allow the page to remain very short, very brief, but still be accurate even if not cutting edge. I would like to thank you both for the opportunity to work on this and apologize ahead of time for not knowing all the text-based vodoo that goes into make the page link correctly. LCDR IAM (talk) 20:57, 13 January 2013 (UTC)
- Those are very promising points, it does sound like a short page might be best for now. May I suggest we move on to the next section? This is a bit of an odd situation, editing in realtime is a little tricky. If my idea below doesn’t work out, we’ll stumble through to another solution. WLU (t) (c) Wikipedia’s rules:simple/complex 21:00, 13 January 2013 (UTC)
- There are a few review articles, but honestly they are a list of the primary sources condensed. I guess I’d argue that multiple trials showing the same thing should be okay – however, I understand and respect your concern. If the issue is that you want no mention of treatment benefits that are not in a textbook, that might be hard and I am willing to leave all that out. Thus, I can remove all of that and just try to focus on clearly stating what is known about the lymphocyte product and that the supplement is not the same thing. I.E. mention that there was evidence of benefit, but none was more than a pre-clinical study and should not be looked upon and a secondary source (I would thus not list any disease specifically). Point out that the science of TF as a immune molecule is on going, but died out years ago. Lastly, point out that the dietary supplement is different and can make no viable claims to being the same thing (which is what I most want to highlight b/c I neither want the molecule to get a bad name from the supplement nor the supplement to ride the coattails of the molecule – even if said coat tails are decades old). This should allow the page to remain very short, very brief, but still be accurate even if not cutting edge. I would like to thank you both for the opportunity to work on this and apologize ahead of time for not knowing all the text-based vodoo that goes into make the page link correctly. LCDR IAM (talk) 20:57, 13 January 2013 (UTC)
-
-
-
Some questions
I’m hoping LCDR IAM can answer these, and build the answers into the page. Since these are separate sections, it’s a little easier to interstitch discussions. WLU (t) (c) Wikipedia’s rules:simple/complex 20:50, 13 January 2013 (UTC)
Most recent review article
What’s the most recent review article on transfer factors? WLU (t) (c) Wikipedia’s rules:simple/complex 20:50, 13 January 2013 (UTC)
- This is the most recent review PMID 18297853. But I would not consider it the best. I believe a lot of their statements, but if I am getting a picture of what sources you want, I doubt it will reach your bar. LCDR IAM (talk) 21:13, 13 January 2013 (UTC)
- The best thing then, is to start from what is included in that source. From there, we can discuss what kinds of lower-bar sources we can use (primary sources in peer-reviewed journals being the next rung down, used judiciously) and what kinds of statements they can verify. WLU (t) (c) Wikipedia’s rules:simple/complex 22:58, 13 January 2013 (UTC)
Supplement versus research molecule
What is the most recent and best source that distinguishes between the two? WLU (t) (c) Wikipedia’s rules:simple/complex 20:50, 13 January 2013 (UTC)
- This is a good question. I’d say it is more of a case of parallel research that unfortunately uses the same term. I can list the sources from which the supplement company is deriving its claims – I have those on my work computer and have to get them. But I hesitate to cite them if you are worried about only having review articles and sources. Finding one that directly compares the two will be difficult since no one has done that. But there is lies my point – since no one has ever directly compared the two, the dietary supplement should not be allowed to make claims that their product in any way is comparable. There have been some that found that the dietary product as similar in vitro effects – but that is truly confined to a petri dish and would be way under your threshold . LCDR IAM (talk) 21:13, 13 January 2013 (UTC)
- When you say «dietary supplement», do you mean something consumed orally, versus the research ones you work with which, I believe, are injected?
- Sources discussing in vitro experiments could be mentioned with great caution, particularly more recent ones.
- Have there been any studies of oral use of TF? WLU (t) (c) Wikipedia’s rules:simple/complex 23:01, 13 January 2013 (UTC)
-
-
- Yes, the dietary supplement is taken orally. 99.9% of research done on cell-derived TF has been injected.
- I will avoid any citation based purely on in vitro.
- There was one study showing the product that is normally injected can be taken orally with similar effects. But the explanation of this study would be well into the weeds compared to what we seem to be aiming for. So, as of now I’d just say it is an injected product only. — Preceding unsigned comment added by LCDR IAM (talk • contribs) 00:38, 14 January 2013 (UTC)
- It would be great if you could find a source that distinguishes between oral and injected, that alone would be very helpful. If you find an in vitro source you think is useful, even though primary, please bring it up on the talk page and we can discuss it. Again, in most cases we avoid primary sources, in some cases we can use them judiciously. I would prefer to run out of secondary before we start though! Something I sometimes do when working with other editors is start a new section on the talk page and fill it with a list of sources that are not integrated but I think are useful. If you are unsure about any, feel free to populate such a section and I will look at anything you put in it. WLU (t) (c) Wikipedia’s rules:simple/complex 01:25, 14 January 2013 (UTC)
-
What are some of the other terms for TF
«Dialyzable leukocyte extract» seems to be one, are there others? How about putting them into the lead, with citations? Usually that would look like this:
Transfer factors, also known as dialyzable leukocyte extract[1] and foo[2] are…
Also note that citation always follow puncutation. It’s always like this.[1] It’s never like this[1], or like this[1]. WLU (t) (c) Wikipedia’s rules:simple/complex 20:50, 13 January 2013 (UTC)
- Understood on the citations. DLE is the only term I have heard to reference cellular TF other than transfer factor. I believe the original articles used something like ‘transfer substance’ but that was pre-1955 and the term TF was started shortly thereafter. The milk/egg based has attempted to usurp prolene-rich polypeptides and I will occasionally see statements about PRP. There are small (primary sourced) studies showing PRP has effects that overlap with milk/egg TF – but there is 0 evidence the two are the same. LCDR IAM (talk) 21:13, 13 January 2013 (UTC)
- I’m not sure what you mean when you say «cellular TF other than transfer factor». Could you clarify?
- Why have they used such a generic name? For that matter, why are all small, low-molecular weight compounds referred to with the same term? Have you seen this book? What about this reference? And for that matter, why did research seem to stop so suddenly, was it solely HIV? That doesn’t seem like the best reason to stop, particularly for a promising substance.
- Actually, that brings up a point I’ll raise in a section below. WLU (t) (c) Wikipedia’s rules:simple/complex 23:14, 13 January 2013 (UTC)
- Sorry – I mean to say that the transfer factor that was made from cells has only gone under the name ‘transfer factor’ or DLE. There should not be any other search term that will refer to the cellular lysate version.
- It is unfortunate that they used such a generic name. But in 1955 that is about all they knew – it ‘transferred’ immunity from one person to another. There were efforts to change to DLE as a term since it was more accurate, but the big wigs writing papers at the time stuck with TF and thus we are stuck with it as well. I don’t think they predicted the absurd claims that would follow from the dietary supplement.
- I have seen those articles. The 1975 book will not offer much more, but I am happy to cite that if it is seen as more of a secondary source. The scandal is a story unto itself. Basically, the research world was lit on fire so to speak and everyone started looking into the possibility that a very small molecule could have immune effects (which was unheard of at the time as IL-1 was not discovered until the 70s). At first it was mostly transfer of skin reaction and swelling, but eventually a researcher at Harvard (big wig at the time) started claiming it could cure Alzheimer’s and Autism. In some of his work he also claimed that TF was a double-stranded RNA molecule. At that time, there was no evidence double stranded RNA existed, so the claim was seen as even blasphemy. It was later discovered that this Harvard researcher was faking his evidence. Burning Guinea Pigs with a lamp and claiming it was an immunologic response. So all his claims of neurologic effects already had TF on shaky ground before his immunologic ones were exposed as fraud. So what followed was a perfect storm leading to the abandonment of transfer factor. First, you have the deflation of all non-immunologic effects, followed by a big wig at Harvard being exposed as a fake. Then, the research into small molecules and immunity started bearing much more productive fruit with the discovery of interleukins – so researchers that wanted to look into such things were better suited doing so in ways other than the tainted TF. Then HIV and Hep C were about the death nail. There was no way anyone would get FDA approval for even the smallest of studies to use a blood product as a treatment (you will note that all studies post about 1980 or so are in Mexico or Europe, some in Japan). Thus, if there was 0% chance of making this into a therapy (i.e. no long term in clinical research), and the basic research was tainted by recent scandal, why would anyone opt to research it? No one really did after that save a few lab-only studies. I hope to restart this work as I think it has been long enough that people should be open to the idea again – especially since there was plenty of evidence that it could have an effect (i.e. the Harvard fraud represented only a small % of the TF research that was out at the time). — Preceding unsigned comment added by LCDR IAM (talk • contribs) 00:53, 14 January 2013 (UTC)
- That would be a profoundly helpful addition to the page, that sort of real-world context for research is one area of wikipedia most chem/biochem articles are lacking but make for salacious reading. Sounds horrible, but think of it as the film that makes you read the book 🙂
- Based on some of the information I have found, I can start editing the page. However, if you would prefer to do so yourself (for practice, or just because you think, probably correctly, you’d do a better job), then I am fine with that. Chances are I would start with the article from the ’70s because that sort of thing doesn’t require any technical expertise. WLU (t) (c) Wikipedia’s rules:simple/complex 01:30, 14 January 2013 (UTC)
- Allow me to make the edits – I should be able to so in just a moment. After you look it over you can feel free to shift things around. I have no doubt in my mind that I will need assistance making the links and everything come out correctly. Then you can just let me know areas that need further repair and I’ll put that in as well. Thanks for your assistance.LCDR IAM (talk) 01:38, 14 January 2013 (UTC)
History section
For an apparently promising, novel substance, TF seems to have dropped off the research radar remarkably efficiently. That suggests something notable in the history of the substance. That might even be a starting point. The threshold for historical claims is lower than for medical claims, and the literature review section found in most article would be readily and unproblematically usable if it discusses the history of TF. Everything still needs to be verifiable, but we can expand on things more readily. WLU (t) (c) Wikipedia’s rules:simple/complex 23:14, 13 January 2013 (UTC)
- I can certainly try to work in the historical aspects. Honestly it will be hard to find published reports detailing all that I laid out above. The New Scientist reference does a good job, but won’t explain the full scale desertion from the research. I’ll try to work it in when I plug everything together. If I overstep or fail just let me know and I’ll continue to clean up as requested.LCDR IAM (talk) 00:59, 14 January 2013 (UTC)
Pseudoscience
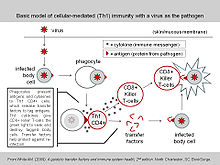
(Note: The image seems to show transfer factors as a kind on antibody produced by CD4+ T cells.)
This article has become a magnet for pseudoscientific claims. I have thus added it in Category:Pseudoscience. All serious scientific research into transfer factor – as «discovered» by Henry Sherwood Lawrence – ended by 1985. Any positive results published after that date are highly questionable and most likely pseudoscience. The article now uses for illustration a graphic by Commons user AaronMatthewWhite from his self published book. The mechanism described, if it ever existed in serious science, has long since been abandoned. — Petri Krohn (talk) 15:09, 9 February 2013 (UTC)
- That seems reasonable. I think it is a bit of an over statement to say that all research since 1985 is pseudoscience in terms of the immune molecule, but it has certainly not been main stream nor in secondary sources. The reason I wanted to separate out the immune molecule from dietary supplement is that ALL supplement claims are pseudoscience and have no evidence (other than that published by the companies selling it – which, of course, is always dubious). Even calling the supplement pseudoscience may be too kind since it is really snake oil with no ‘science’ behind it at all. Whereas there is scientific literature on the effects of the immune molecule and I’d say stopped closer to 2001 (before restarting recently) rather than 1985.
- I’d concur that the drawing from White’s book also is a stretch since I don’t see any hard evidence of the exact mechanism that he illustrates. However work on the immune molecule (while not common) has been on going and in 2001 there were interesting (and serious) papers published on the TF molecule as discovered by Lawrence (albeit again, primary sources only). Others since have been less interesting, but still published in peer-reviewed journals, which I would think it the measure of ‘serious’ in most cases. Lots of items were ‘abandoned’ in medical literature for a while before rediscovery. Ignaz Semmelweis discovered hand washing saved lives decades before it was accepted. So while I think you are correct to label TF with a pseudoscience label to warn people against outlandish claims, I am not sure it is fair to imply all published papers on the immune molecule are fraudulent.
- If the supplement were made into a new article, wouldn’t it have to be basically «this is sold under claims of being related to TF:immune molecule, but no evidence exists» or something along those lines? We can separate them, but the supplement would be no more than a few sentences no? LCDR IAM (talk) 21:37, 9 February 2013 (UTC)
-
- Proving that transfer factor is bogus would be almost a as difficult as disproving cold fusion. I agree that there was great interest and serious research into the topic before 1985. What stopped it may have [been] a greater general understanding of cell-mediated immunity. There is no way you can squeeze anything pathogen-specific into 5 kDa. I see there are similar snake-oil articles in Japanese and Korean. — Petri Krohn (talk) 11:05, 10 February 2013 (UTC)
-
-
- The MHC class II molecule only displays pathogenic proteins in 15-24 amino acid increments. The CDR3 region of both the T and B cell receptors (the part that actually binds to the pathogenic/antigenic epitope) is typically only 7 to 11 amino acids long. Therefore I would put forth that you can pack an amazing amount of epitope specificity into a relatively small amount of molecular investment. Of course, simply binding the epitope would not be enough; you would need an additional end of the molecule to signal through an immunologic receptor and thus the myriad of other explanations must be considered more likely for the results found in transfer factor research. However, while it is absolutely true that until somebody isolates and clones the transfer factor molecule it cannot be considered anything more than antiquated phenomenology, it is equally true that until somebody recreates the findings previously published and proves their own alternative explanation for the results – responding to the published literature (as cob-webbed as it may be) with a backhanded dismissal is not justifiable. LCDR IAM (talk) 21:35, 10 February 2013 (UTC)
-
May I add that what I heard was that Transfer Factor research stopped in the early 1980s because of the onset of HIV and AIDS and so manufacture or Dialyzable leukocyte extract was dangerous. Then, when it was found in the late 1990s that cow colustrum contained the right stuff, it was started again. I do not have any «scientific» evidence that it works, but I have personally seen two instances where it sure looked like Transfer Factor was at least in part responsible for some pretty amazing results in reducing tumors. BTW, I heard about TF from my veterinarian quite a few years ago and take it myself after having 3 types of cancer in the last 15 years but none since I started taking it about 6 years ago. This is my unscientific addition to this subject. I have a book from the 1970s called Clinical Immunology but am not sure if it is in the list of references.boB K7IQ (talk) 23:54, 29 November 2016 (UTC)
Can we get rid of esoteric language in the introduction of the article? It plays right into the hands of MLMs scams like 4Life? I am talking about this: “They are an ancient part of the immune system and represent «an archaic dialect in the language of cells.»” The source doesn’t seem to be publicly available, so I can’t check if it is valid, but even if it is it doesn’t seem appropriate for the introduction of a Wikipedia article. Googling that quote actually turns up mostly 4Life pages. For example on this 4Life page they are even linking to this article, so they are using this article to promote their bullshit. Maybe instead of confusing with esoterik language the introduction should inform the reader that there are currently no medical uses of transfer factors. Jan Path (talk) 15:46, 10 April 2019 (UTC)
|
|
Physicians’ Desk Reference |
…
Factores de ransferencia en el Libro «PDR» de los medicos
Los productos 4Life Transfer Factor® en el PDR del 2015
Salt Lake City, Utah (10 de febrero de 2015) Los productos 4Life Transfer Factor se incluyen, por onceavo año consecutivo, en el Physicians’ Desk Reference (PDR).
La edición del 2015 provee información detallada sobre 4Life Transfer Factor® Tri-Factor® Formula y una descripción técnica de la línea de productos Tri-Factor® Formula. Adicionalmente, la publicación provee una sinopsis de varios estudios científicos en referencia a los productos 4Life. Este año también incluye la fotografía de 4Life Trasnfer Factor Tri-Factor Formula.
“Estamos encantados porque de nuevo han sido incluidos en esta prestigiada publicación”, comentó el Vicepresidente de Mercadotecnia y Desarrollo de Productos Chad Renshaw. “El PDR es uno de los medios de consulta mejor corroborados entre la comunidad médica. Se distribuye a 500,000 profesionales de la salud en todo Estados Unidos. El que los productos 4Life se incluyan en el PDR provee insuperable credibilidad a nuestros distribuidores cuando comparten los productos con sus clientes”.
Con nueve patentes internacionales y docenas más en trámite, un equipo interno de Investigación y Desarrollo, y la experiencia científica del Consejo de Ciencias Médicas, el compromiso de 4Life con la ciencia de los factores de transferencia, no tiene paralelo.
El Director de Servicios de Información de la Salud Brent Vaughan, PhD: “La lista del PDR refuerza nuestra credibilidad científica y empodera a los distribuidores con una valiosa herramienta para compartir los productos 4Life”.
4Life cuenta con oficinas en cinco continentes para dar servicio a una red mundial de distribuidores independientes a través de la ciencia, el éxito y el servicio.
Para más información:
Calvin Jolley
Vicepresidente de Comunicaciones
4Life Transfer Factor Products in 2012 PDR
(Last Updated On: February 28, 2015)
Salt Lake City, Utah (February 7, 2012) 4Life executives are pleased to announce that 4Life Transfer Factor® products are listed in the 2012 Physician’s Desk Reference (PDR).
“We are thrilled to be part of this prestigious and valuable publication. This is the eighth year that 4Life products have been listed,” stated Director of Product Development Chad Renshaw. “The PDR is among the most substantiated resources among the medical community and is distributed to 475,000 health professionals across the United States. Having 4Life products listed provides distributors with unrivaled credibility when presenting our products to customers.”
The 2012 edition of the PDR provides in-depth product information about 4Life Transfer Factor® and 4Life® Transfer Factor Plus® Tri-Factor®Formulas. In addition, the publication offers a technical description of the product line and synopses on several clinical studies.

4Life’s commitment to Transfer Factor science is well established with nine worldwide patents and dozens of patents pending, an in-house Research and Development team, the scientific input of a Health Sciences Advisory Board, and the industry’s only immunological testing lab.
Director of Health Information Systems Brent Vaughan: “This professional listing gives 4Life Transfer Factor an additional boost in the scientific community, and helps strengthen every distributor’s position as an exclusive leader in the distribution of Transfer Factor products.”
4Life has offices on five continents to service a global network of independent distributors through science, success, and service.
¿Qué es el Physicians’ Desk Reference?
El PDR es la guía de referencia para cientos de miles de profesionales de la salud a través de los Estados Unidos. Este libro es la fuente de información de los medicamentos, suplementos alimenticios y hierbas que están disponibles en el mercado actualmente. También provee importante información técnica de las innovaciones en los tratamientos, la ciencia del antienvejecimiento, estudios científicos y nutrición, entre otras cosas más.
¿Cuántos años ha estado en circulación el PDR?
El PDR ha sido publicado por más de 60 años.
¿Cuántos consultorios médicos tienen el PDR?
Hoy en día, el PDR puede encontrarse prácticamente en cada consultorio médico, hospital y farmacia en los Estados Unidos. ¡Este libro se distribuye en 475,000 consultorios médicos! De hecho, nueve de diez médicos consideran el PDR como su mayor fuente de consulta para información de medicamentos y tratamientos.
¿Cuántos años han aparecido los productos de 4Life en el PDR?
Desde principios del 2003, los productos 4Life Transfer Factor® han sido incluidos en el Physicians’ Desk Reference for Nonprescription Drugs, Dietary Supplements, and Herbs. Esta vez, se ha incluido 4Life Transfer Factor Tri-Factor Formula en el PDR principal, que es distribuido a doctores, hospitales y profesionales de la salud en todos los Estados Unidos.
¿Cuál es el contenido de la información de 4Life en el 2011?
La información de 4Life Transfer Factor® Tri-Factor® Formula en el PDR del 2011 incluye una definición completa de los factores de transferencia y sus antecedentes, además de hitos en la investigación. También incluye información importante acerca de la producción de 4Life Transfer Factor Tri-Factor Formula, una lista de varios productos 4Life Transfer Factor, y una sinopsis de diferentes estudios clínicos.
¿De qué forma promueve la credibilidad de los constructores de negocio de 4Life el PDR?
Por más de una década, 4Life ha demostrado su firme compromiso en la fabricación de productos altamente eficaces con un respaldo científico. 4Life tiene una larga historia como parte del Physicians’ Desk Reference for Nonprescription Drugs, Dietary Supplements, and Herbs.
Esta nueva aparición en el PDR principal remarca la buena reputación de 4Life en la industria de los suplementos alimenticios y nuestra dedicación a crear productos que son respaldados por una investigación científica sólida. También refuerza tu posición como líder exclusivo en la distribución de los productos 4Life Transfer Factor. Utilízalo la próxima vez que contactes a un profesional de la salud para hablar de los productos.